Why LOINC Codes Matter in Clinical Trials and Why Local Labs Need Help
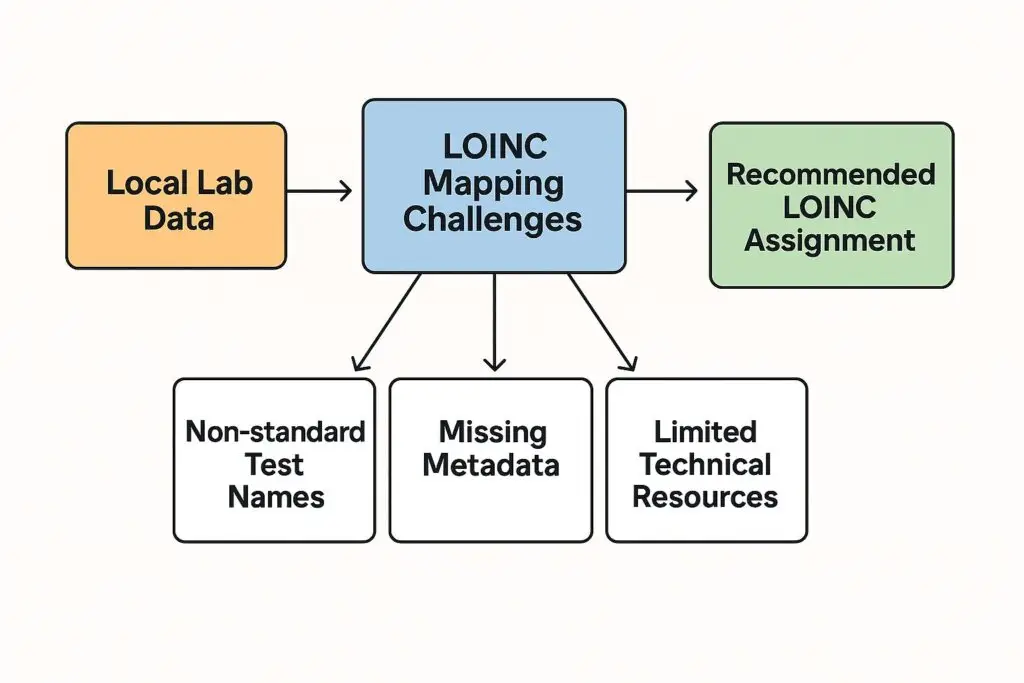
October 12, 2025 5:12 pm In the world of clinical trials, data standardization is more than a technical preference—it’s a regulatory expectation. One of the key standards for lab data is LOINC® (Logical Observation Identifiers Names and Codes), a universal coding system used to identify laboratory tests and clinical observations. The FDA has recommended—and in […]
Why LOINC Codes Matter in Clinical Trials and Why Local Labs Need Help Read More »