- October 12, 2025
- 5:12 pm
In the world of clinical trials, data standardization is more than a technical preference—it’s a regulatory expectation. One of the key standards for lab data is LOINC® (Logical Observation Identifiers Names and Codes), a universal coding system used to identify laboratory tests and clinical observations. The FDA has recommended—and in many cases, expects—LOINC codes to be included in regulatory submissions for clinical trial data, particularly within the Laboratory (LB) domain of CDISC-compliant datasets. [fda.gov]
But here’s the challenge: many local or decentralized labs still don’t use LOINC codes. This gap can create significant hurdles for sponsors and CROs trying to ensure their submissions meet FDA expectations.
Why Local Labs Struggle with LOINC Implementation
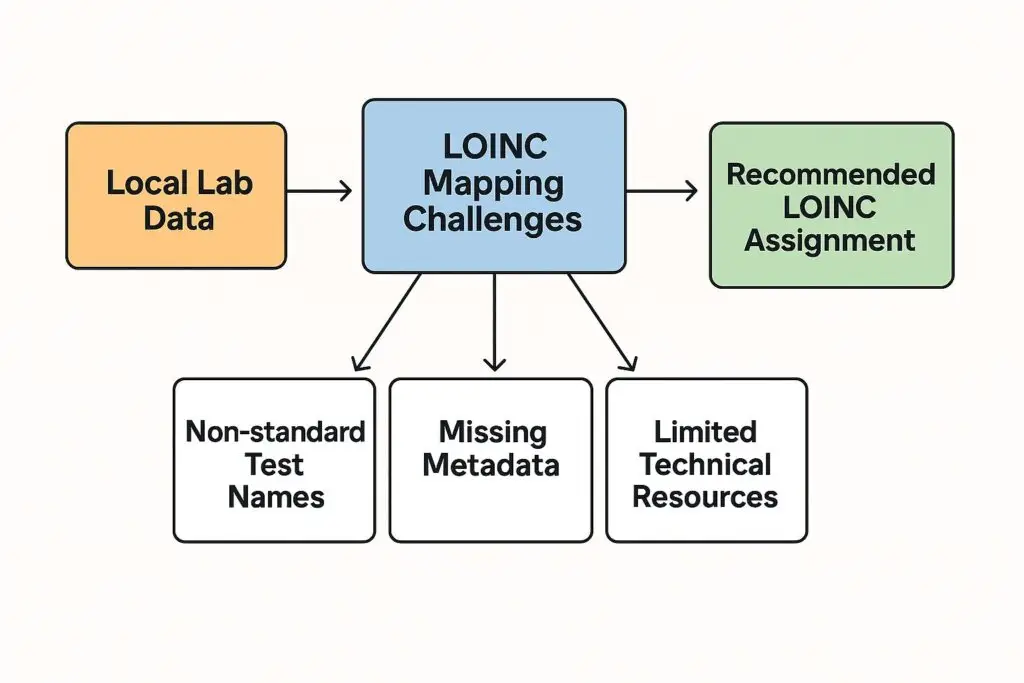
Local labs often operate outside the infrastructure of large central labs or hospital systems. They may use custom test menus, lack standardized naming conventions, or rely on legacy systems that don’t support LOINC mapping. Some of the most common issues include:
- Non-standard test names that don’t clearly map to a LOINC code.
- Missing metadata (e.g., specimen type, method, timing) needed to assign a precise LOINC.
- Limited technical resources to implement LOINC mapping or update lab information systems.
- Lack of awareness about regulatory expectations for LOINC in clinical research. [meridian.a…npress.com]
Even when labs attempt to assign LOINC codes, the process can be resource-intensive and error-prone, requiring careful interpretation of test details and manual review. [lhncbc.nlm.nih.gov]
FDA’s Position on LOINC in Submissions
The FDA’s guidance encourages sponsors to include LOINC codes for lab test results when available. If a lab cannot provide a LOINC code, sponsors are expected to make a reasonable effort to assign one or explain the omission in the Study Data Reviewer’s Guide (SDRG). The goal is to improve interoperability, clarity, and consistency in lab data across studies and submissions. [cdisc.org]
How Our Service Helps
At Lab Data Solutions LLC, we specialize in helping clinical trial teams bridge the gap between local lab data and regulatory expectations. Our LOINC Audit Review service offers:
- Expert review of local lab datasets to identify missing or incorrect LOINC codes.
- Recommended LOINC assignments based on test name, specimen type, method, and other metadata.
- Compliance checks to ensure your lab data aligns with FDA guidance and CDISC standards.
- Documentation support for SDRG entries when LOINC codes cannot be assigned.
Whether you’re working with a small specialty lab or managing data from multiple decentralized sites, we help ensure your submission is LOINC-complete and FDA-ready.
Let’s Make Your Lab Data Submission-Ready
If you’re preparing a regulatory submission and need help with LOINC compliance, reach out to us. We’ll help you navigate the complexities and ensure your data meets the standards that regulators expect.